Answer:
When the temperature of the gas is increased from 20 to 40, the pressure will be 2p
Step-by-step explanation:
Given;
initial temperature of the gas, T₁ = 20 K
final temperature of the gas, T₂ = 40 k
initial pressure of the gas, P₁ = P
final pressure of the gas, P₂ = ?
Apply pressure law of gases;
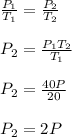
Therefore, when the temperature of the gas is increased from 20 to 40, the pressure will be 2p