Answer:
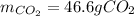
Step-by-step explanation:
Hello,
In this case, considering the combustion of propane:
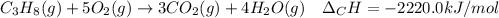
We can compute the burnt moles of propane as shown below:
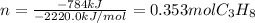
Then, by noticing propane and carbon dioxide are in a 1:3 molar ratio, we can compute the grams carbon dioxide by using the shown below stoichiometric procedure:
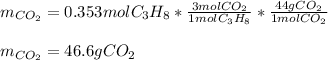
Best regards.