Answer:
d. A weak base
Step-by-step explanation:
For this question, we have to start with the reaction between
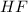 and
and
 . So:
. So:
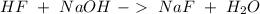
Now, we have to analyze each compound:
Fluorhydric acid HF this is a strong acid because it has the ability to produce hydronium ions

Sodium hydroxide NaOH this is a strong base because it has the ability to produce hydroxide ions

Sodium fluoride NaF is a salt in which the cation is
 and the anion is
and the anion is
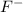
Water Is a neutral molecule usually the solvent
Now, we have the reaction of a strong acid with a strong base. If we remember the conjugated base theory, a strong acid will produce a weak base. So, NaF is a weak base. We also have water, but this is a neutral molecule. With this in mind, the answer is d.
I hope it helps!