Answer:
A. 605 mL of the 35% solution
B. 55 mL of the 95% solution
Explanation:
It is given that, you need 660 mL of a 40% alcohol solution. On hand, you have 35% alcohol mixture. You also have a 95% alcohol mixture.
Let x and y be the amount of 35% alcohol solution and 95% alcohol solution (in mL) respectively.
So,
Amount equation:
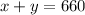 ...(1)
...(1)
Alcohol equation :
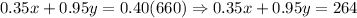 ...(2)
...(2)
On solving (1) and (2), we get
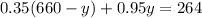
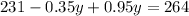
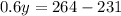
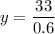
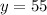
Now, substitute y=55 in (1).
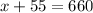
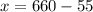
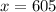
Therefore, we need 605 mL of the 35% solution and 55 mL of the 95% solution.