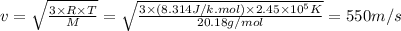Answer:
550 m/s
Step-by-step explanation:
The average molecular speed (v) is the speed associated with a group of molecules on average. We can calculate it using the following expression.
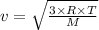
where,
We can use the info of argon to calculate the temperature for both samples.
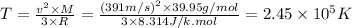
Now, we can use the same expression to find the average molecular speed in a sample of Ne gas.