Answer:
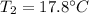
Step-by-step explanation:
Hello,
In this case, we can solve this problem by noticing that the heat lost by the warm water is gained by the ice in order to melt it:
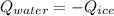
In such a way, the cooling of water corresponds to specific heat and the melting of ice to sensible heat and specific heat also that could be represented as follows:

Thus, specific heat of water is 4.18 J/g°C, heat of melting is 334 J/g and specific heat of ice is 2.04 J/g°C, thus, we can compute the final temperature as shown below:
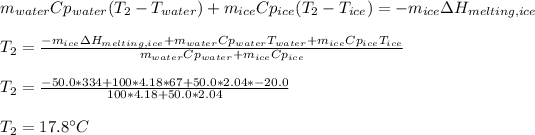
Best regards.