Answer:
a) efficiency is equal to 67.2%, and this is lesser than the maximum obtainable efficiency, so this power output is possible.
b) efficiency is 75%, this is approximately equal to the maximum obtainable efficiency, but not more that it. This power output is also possible.
Step-by-step explanation:
Cold reservoir temperature Tc = 295 K
Hot reservoir temperature Th = 1175 K
Energy input Q = 150000 K/h
Converting to kJ/s, Q = 150000/3600 = 41.66 kJ/s
Maximum efficiency that can be obtained from this cycle =
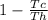
==>
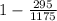 = 0.748 ≅ 75%
= 0.748 ≅ 75%
also recall that actual cycle efficiency =
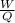
Where W is the energy output or work
a) for work of 28 kW,
eff =
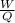 =
=
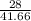 = 0.672 ≅ 67.2%
= 0.672 ≅ 67.2%
this is lesser than the maximum obtainable efficiency, so this power output is possible.
b) for work of 31.2 kW
eff =
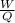 =
=
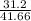 = 0.748 ≅ 75%
= 0.748 ≅ 75%
this is approximately equal to the maximum obtainable efficiency, but not more that it. This power output is possible.
NB: kW is also equal to kJ/S