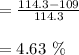Answer:
Following are the answer to this question:
Step-by-step explanation:
In the given question an attachment file is missing, that is attached. please find the attached file, and the following are the description of the given points:
a. At 100 degrees in 100 mL 5 g is dissolved.
For, it required:
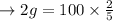
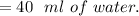
b. At 0 degrees 100 mL dissolve in 0.3 g.
So, the dissolve:
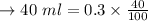
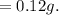
After refrigeration 0.12 g will still be dissolved.
c. After dissolving and freezing, precipitation can occur which would still be impure if the cooling is instantaneous. The added solvent was also too hard to recrystallize. The solvent was placed below its place of reservation.
d. Recovery percentage:
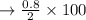
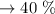
The melting point of acetanilide:
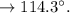
Found=109(medium)
Melting point error percentages: