Answer:
9.308
Step-by-step explanation:
The computation of the pH of the given solution is shown below:
But before we need to determine the HI molarity which is
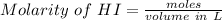
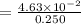
= 0.1852 M
Now
As we know that
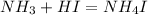
So,
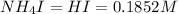
Now the molarity of
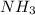 left is
left is
= 0.397 - 0.1852
= 0.2118
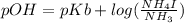
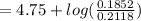
= 4.692
Now as we know that
pH = 14 - pOH
= 14 - pOH
= 14 - 4.692
= 9.308
We simply applied the above equations