Answer:
total time = 304.21 s
Step-by-step explanation:
given data
y = 50% = 0.5
n = 1.1
t = 114 s
y = 1 - exp(-kt^n)
solution
first we get here k value by given equation
y = 1 -
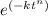 ...........1
...........1
put here value and we get
0.5 = 1 - e^{(-k(114)^{1.1})}
solve it we get
k = 0.003786 = 37.86 ×
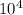
so here
y = 1 -
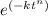
1 - y =
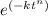
take ln both side
ln(1-y) = -k ×
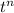
so
t =
![\sqrt[n]{-(ln(1-y))/(k)}](https://img.qammunity.org/2021/formulas/engineering/college/knab1at9z5cyjavji97tsfq8vrcxswcpye.png) .............2
.............2
now we will put the value of y = 87% in equation with k and find out t
t =
![\sqrt[1.1]{-(ln(1-0.87))/(37.86*10^(-4))}](https://img.qammunity.org/2021/formulas/engineering/college/10lxg9hl4ojo41aelm9lkqngs8fmnz3512.png)
total time = 304.21 s