Answer:
Following are the answer to this question:
Step-by-step explanation:
In the given question information is missing, that is equation which can be defined as follows:
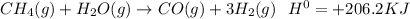
- Growing temperatures may change its connection to just the way which consumes thermal energy in accordance with Le chatelier concepts Potential connection is endothermic. Answer: shifts to the right
- Kc are described as a related to the concentration by the intensity of both the reaction for each phrase which reaches a power equal towards its stoichiometric equation coefficient Kc = \frac{product}{reactant} It increases [product] but reduces [reactant] Therefore, Kc increases