Answer:
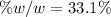
Step-by-step explanation:
Hello,
In this case, since the solution is formed by sodium chloride and water, we understand that for one mole of solution, we have 0.132 moles of sodium chloride and 0.868 moles of water, therefore, by using their molar masses we can compute their masses in the solution:
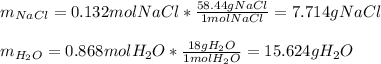
Thereby, we can compute the weight/weight percent as follows:
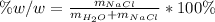
So we use the previously computed masses:
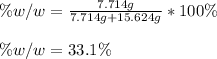
Best regards.