Answer: The ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.
Step-by-step explanation:
Law of constant proportion states that In a chemical substance the elements are always present in definite proportions by mass. This law is also known as 'Law of definite proportions '.
Mass of 1 atom of sulphur = 32 g
Mass of 1 atom of oxygen = 16 g
Mass of 2 atoms of oxygen =
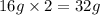
In formation of
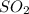 , 1 atom of sulfur combines with 2 atoms of oxygen and thus the mass ratio will be 32: 32= 1:1 .
, 1 atom of sulfur combines with 2 atoms of oxygen and thus the mass ratio will be 32: 32= 1:1 .
Thus the ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.