Hey there!
For this we can use the combined gas law:
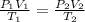
We are only working with pressure and temperature so we can remove volume.
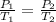
P₁ = 2 atm
T₁ = 27 C
P₂ = 2.2 atm
Plug these values in:
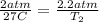
Solve for T₂.
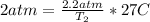
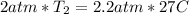
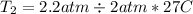
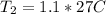
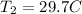
Convert this to kelvin and get 302.85 K, which is closest to B. 330 K.
Hope this helps!