Answer:
The half-life of the radioactive isotope is 346 years.
Step-by-step explanation:
The decay rate of the isotope is modelled after the following first-order linear ordinary differential equation:
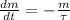
Where:
 - Current isotope mass, measured in kilograms.
- Current isotope mass, measured in kilograms.
 - Time, measured in years.
- Time, measured in years.
 - Time constant, measured in years.
- Time constant, measured in years.
The solution of this differential equation is:
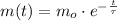
Where
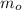 is the initial mass of the isotope. It is known that radioactive isotope decays at a yearly rate of 0.2 % annually, then, the following relationship is obtained:
is the initial mass of the isotope. It is known that radioactive isotope decays at a yearly rate of 0.2 % annually, then, the following relationship is obtained:
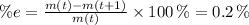
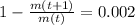
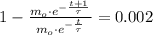
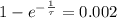
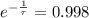
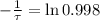
The time constant associated to the decay is:
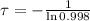
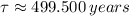
Finally, the half-life of the isotope as a function of time constant is given by the expression described below:
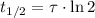
If
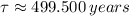 , the half-life of the isotope is:
, the half-life of the isotope is:
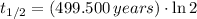
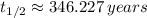
The half-life of the radioactive isotope is 346 years.