Answer:
Ecell = +0.25V
Step-by-step explanation:
the half-cell reactions for a voltanic cell
cathode(reduction): 2H⁺(aq) + 2e⁻ ------- H₂(g)
anode(oxidation): 2AgCl(s) ------- 2Ag⁺(aq) + 2Cl⁻ + 2e⁻
we have the standard cell potential E⁺cell = 0.18V at 80C respectively
Q = [H⁺]/[Cl⁻]
sub for [H+] = 0.10M and [Cl-] = 1.5M
Q= 0.1M/1.5M
Q = 0.067
Ecell = E⁺cell -
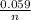 logQ
logQ
= 0.18 -
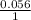 log 0.067
log 0.067
0.18- 0.059(-1.174)
Ecell = +0.25V