Let us take a look at the known values, or the " given. " C and D stand for concentrated and diluted in this case -
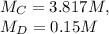
Given this, let us say that the " original concentrated volume " is x. If so, the " original diluted volume " is x + 100 -
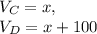
_______________________________________________________
Using the formula "
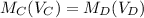 " solve for x, the volume of the original sample,
" solve for x, the volume of the original sample,
Mc( Vc ) = Md( Vd ),
3.817( x ) = 0.15( x + 100 ),
3.817x = 0.15x + 15,
3.667x = 15,
x = ( About ) 4.09
( The volume of the original sample is about 4.09 mL )