Answer:
![[ICI]_(eq)=0.271M](https://img.qammunity.org/2021/formulas/chemistry/college/pm4ie0wdawgjgwwqoa4pomowqglpjqt9e1.png)
Step-by-step explanation:
Hello,
In this case, considering that the equilibrium constant for the following reaction:
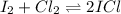
Is Kc=9.09, we can compute the equilibrium concentrations by the ICE procedure, so we first compute the initial concentrations:
![[I_2]_0=[Cl_2]_0=(0.45mol)/(2.0L)= 0.225M](https://img.qammunity.org/2021/formulas/chemistry/college/hyr26jjib3ocka709tgorm0m191gki4slt.png)
Next, we write the law of mass action in terms of the change
 due to the reaction extent:
due to the reaction extent:
![Kc=([ICI]^2)/(([I_2])([CI_2])) \\\\Kc=((2x)^2)/(([I_2]_0-x)([CI_2]_0-x)) \\\\9.09=((2x)^2)/((0.225-x)(0.225-x))](https://img.qammunity.org/2021/formulas/chemistry/college/v5q3ywfl2ajhwo3o7xur6f2d00ogxpp6s9.png)
Thus, solving by using solver we have:
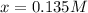
Therefore, equilibrium concentration of ICl is:
![[ICI]_(eq)=2*0.135M](https://img.qammunity.org/2021/formulas/chemistry/college/rdup8oeh9x3mp2x9mud9u9oavizrowyw5m.png)
![[ICI]_(eq)=0.271M](https://img.qammunity.org/2021/formulas/chemistry/college/pm4ie0wdawgjgwwqoa4pomowqglpjqt9e1.png)
Regards.