Complete Question
A student is extracting caffeine from water with dichloromethane. The K value is 4.6. If the student starts with a total of 40 mg of caffeine in 2 mL of water and extracts once with 6 mL of dichloromethane
The experiment above is repeated, but instead of extracting once with 6 mL the extraction is done three times with 2 mL of dichloromethane each time. How much caffeine will be in each dichloromethane extract?
Answer:
The mass of caffeine extracted is
Step-by-step explanation:
From the question above we are told that
The K value is
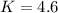
The mass of the caffeine is
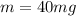
The volume of water is
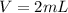
The volume of caffeine is
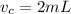
The number of times the extraction was done is n = 3
Generally the mass of caffeine that will be extracted is
![P = m * [(V)/(K * v_c + V) ]^3](https://img.qammunity.org/2021/formulas/chemistry/college/xpsy8ocbsrswhz87k9fw7lt770p0s6l1bv.png)
substituting values
![P = 40 * [(2)/(4.6 * 2 + 2) ]^3](https://img.qammunity.org/2021/formulas/chemistry/college/sdgz20au0gstl80g6hnl0131emzpj6g01q.png)