Answer:
Copper (II) chloride.
Step-by-step explanation:
Hello,
In this case, considering the described reaction which is also given as:
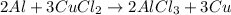
For us to identify the limiting reactant we first compute the available moles of aluminium:
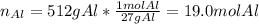
Next, we compute the consumed moles of aluminium by the 1147 grams of copper (II) chloride by using their 2:3 molar ratio:
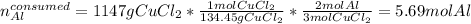
Thereby, we can infer aluminium is in excess since less moles are consumed than available whereas the copper (II) chloride is the limiting reactant.
Best regards.