Answer:
a) 2-bromopyrrole
Step-by-step explanation:
Our options for this questions are:
a) 2-bromopyrrole
b) 2,3-dibromopyrrole
c) N-bromopyrrole
d) 3-bromopyrrole
To understand how the reaction works we have to start with the resonance structures. (Figure 1), on these structures, we will obtain a negative charge on carbon 2 in the pyrrole ring, therefore on this carbon we can generate an attack to an electrophile.
The second step is to check how the mechanism take place. An electrophile is generated by the
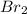 and
and
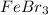 . This electrophile can be attacked by the negative charge on carbon 2 producing the 2-bromopyrrole. (See figure 2).
. This electrophile can be attacked by the negative charge on carbon 2 producing the 2-bromopyrrole. (See figure 2).
I hope it helps!