Question:
A 10 gauge copper wire carries a current of 15 A. Assuming one free electron per copper atom, calculate the drift velocity of the electrons. (The cross-sectional area of a 10-gauge wire is 5.261 mm².)
Answer:
3.22 x 10⁻⁴ m/s
Step-by-step explanation:
The drift velocity (v) of the electrons in a wire (copper wire in this case) carrying current (I) is given by;
v =
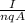
Where;
n = number of free electrons per cubic meter
q = electron charge
A = cross-sectional area of the wire
First let's calculate the number of free electrons per cubic meter (n)
Known constants:
density of copper, ρ = 8.95 x 10³kg/m³
molar mass of copper, M = 63.5 x 10⁻³kg/mol
Avogadro's number, Nₐ = 6.02 x 10²³ particles/mol
But;
The number of copper atoms, N, per cubic meter is given by;
N = (Nₐ x ρ / M) -------------(ii)
Substitute the values of Nₐ, ρ and M into equation (ii) as follows;
N = (6.02 x 10²³ x 8.95 x 10³) / 63.5 x 10⁻³
N = 8.49 x 10²⁸ atom/m³
Since there is one free electron per copper atom, the number of free electrons per cubic meter is simply;
n = 8.49 x 10²⁸ electrons/m³
Now let's calculate the drift electron
Known values from question:
A = 5.261 mm² = 5.261 x 10⁻⁶m²
I = 23A
q = 1.6 x 10⁻¹⁹C
Substitute these values into equation (i) as follows;
v =
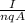
v =
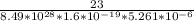
v = 3.22 x 10⁻⁴ m/s
Therefore, the drift electron is 3.22 x 10⁻⁴ m/s