1) The metal which reduces the other compound is the one higher in the reactivity. So in this case it is
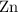 .
.
2) The substance which brings about reduction while itself getting oxidised (that is losing electrons) is called a reducing agent. Here, $\mathrm{Zn}$ is the reducing agent and reduces Cobalt Oxide to Cobalt while itself getting oxidised to Zinc oxide.