Answer: 290 g of aluminium sulphate is produced.
Step-by-step explanation:
To calculate the moles :
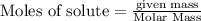
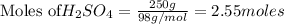
The balanced chemical reaction is:
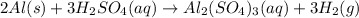
According to stoichiometry :
3 moles of
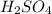 produce = 1 mole of
produce = 1 mole of
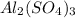
Thus 2.55 moles of
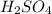 will require=
will require=
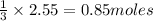 of
of
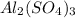
Mass of
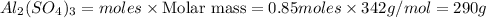
Thus 290 g of aluminium sulphate is produced.