Answer:
See the explanation and answer below.
Step-by-step explanation:
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. The formula gives the proportions of the elements present in a compound but not the actual arrangement of atoms.
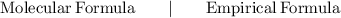
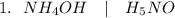 (Ammonium hydroxide)
(Ammonium hydroxide)
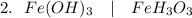 (Iron(III) hydroxide)
(Iron(III) hydroxide)
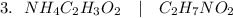 (Ammonium acetate)
(Ammonium acetate)
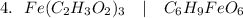 (Iron(III) Acetate)
(Iron(III) Acetate)
I hate chemistry but best regards!