Answer: Thus the % yield is 86.2%
Step-by-step explanation:
Percentage yield is the ratio of actual change to the theoretical change in terms of percentage.
The given reaction is :
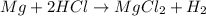
To calculate the percent yield :
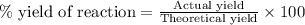
Given : Actual pressure increase = 0.25 atm
Theoretical pressure change = 0.29 atm.
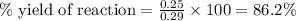
Thus the % yield is 86.2%