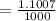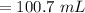Answer:
The correct answer will be "100.7 mL". The further explanation is given below.
Step-by-step explanation:
The given values are:
Temperature,
ΔT = 1°C
Mass,
m = 70 kg
c = 3.480 J/Kg.K
Amount of released heat will be:
⇒
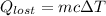
On putting the estimated values, we get
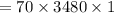
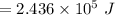
Let M will be the amount of evaporated water at the temperature of 37°C.
Required heat will be:
⇒
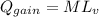
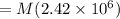
Now, Lost heat will be equal to the required amount of heat.
⇒
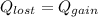
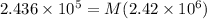
On applying cross-multiplication, we get
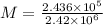
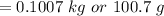
Now,
⇒
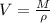
On putting the estimated values, we get