Answer:
4.3
Step-by-step explanation:
Data provided in the question as per the question is as follows
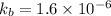
Now we use the log in both the sides
So,
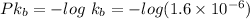
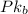 = 5.795
= 5.795
And, C = 0.0015
log c = -2.823
Now pH is
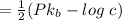
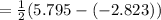
= 4.3
Hence, the pH of the solution of 0.0015 M morphine is 4.3 and the same is to be considered by applying the above formulas and the calculations part