Answer:
The reaction is not in equilibrium
Step-by-step explanation:
For the reaction:
PCl₅ ⇄ PCl₃ + Cl₂
Equilibrium constant, Kp, is defined as:
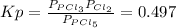
When this ratio is = 0.497, the reaction is in equilibrium. Replacing the pressures of the problem, reaction quotient, Q, is:
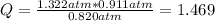
As Q ≠ Kp, the reaction is not in equilibrium
To reach the equilibrium, the reaction will shift to the left producing more reactant and decreasing amount of products.