Answer:
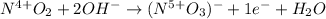
Step-by-step explanation:
Hello,
In this case, for the given reaction, we first start by the writing of the oxidation states of all the involved elements:
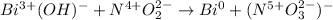
In such a way, we are noticing nitrogen is undergoing an increase in its oxidation state, therefore it is being the oxidized species, for which the oxidation half reaction, should be (considering basic conditions):
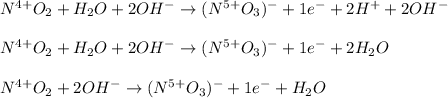
Best regards.