Answer:
To the right
Step-by-step explanation:
Step 1: Given data
- Partial pressure of PCl₅ (pPCl₅) = 0.548 atm
- Partial pressure of PCl₃ (pCl₃) = 0.780 atm
- Partial pressure of Cl₂ (pCl₂) = 0.780 atm
Step 2: Write the balanced equation
PCl₅(g) ⇄ PCl₃(g) + Cl₂(g)
Step 3: Calculate the pressure reaction quotient
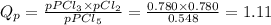
Step 4: Determine whether the reaction proceeds to the right or to the left as equilibrium is approached
Since Qp < Kp, the reaction will proceed to the right to attain the equilibrium.