Answer:
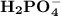 and
and
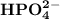 are the amphoteric species.
are the amphoteric species.
Step-by-step explanation:
Phosphorus is a non-metallic element belonging to Group 5 of the Periodic table. As a triprotic acid (i.e an acid that has three dissociable protons that undergo stepwise ionization) . Phosphorus reacts vigorously with hot water to form tetraoxosulphate(V) acid.
i.e
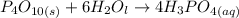
SO;
As a triprotic acid;
The ionization equations are as follows:
NOTE: Instead of the amphoteric species to be circle as instructed , due to the fact that we are using the LaTex Editor ; we will just need to let them be in bold format which will differentiate them from the rest.
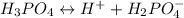
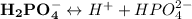
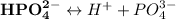
Thus;
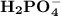 and
and
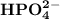 are the amphoteric species.
are the amphoteric species.