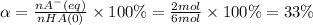Answer:
33 %
Step-by-step explanation:
Step 1: Given data
Initial moles of the acid (nHA(0)): 6 mol
Moles of the conjugate base at equilibrium (nA⁻(eq))
Step 2: Write the balanced generic acid dissociation reaction
HA(aq) ⇄ A⁻(aq) + H⁺(aq)
Step 3: Calculate the percent ionization
We will use the following expression.