Answer: There are
 formula units
formula units
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
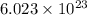 of particles.
of particles.
To calculate the moles, we use the equation:
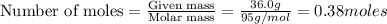
1 mole of
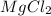 contains =
contains =
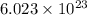 formula units
formula units
Thus 0.38 moles of
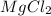 contains =
contains =
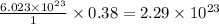 formula units
formula units
Thus there are
 formula units
formula units