Answer:
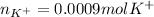
Step-by-step explanation:
Hello,
In this case, the first step is to compute the number of moles of potassium phosphate in 20.0 mL (0.020L) of the 0.015-M (mol/L) solution as shown below:
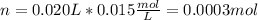
Thus, these moles correspond to potassium phosphate moles, which molecular formula is K₃PO₄, therefore, one mole of this compound contains three moles of potassium ions as it has three as its subscript in the formula. Thereby, the moles of potassium ions result in:
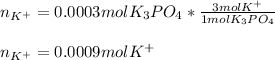
Best regards.