Step-by-step explanation:
It is given that,
The electron in a hydrogen atom, originally in level n = 8, undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm. It means that,
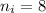
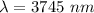
The amount of energy change during the transition is given by :
![\Delta E=R_H[(1)/(n_f^2)-(1)/(n_i^2)]](https://img.qammunity.org/2021/formulas/chemistry/college/3qci4p1suuyl0vyaef3ogwz8zlm20gfmlk.png)
And
![(hc)/(\lambda)=R_H[(1)/(n_f^2)-(1)/(n_i^2)]](https://img.qammunity.org/2021/formulas/chemistry/college/hiancu46e187yxp2lvzv4nkdefqz0411fk.png)
Plugging all the values we get :
![(6.63* 10^(-34)* 3* 10^8)/(3745* 10^(-9))=2.179* 10^(-18)[(1)/(n_f^2)-(1)/(8^2)]\\\\(5.31* 10^(-20))/(2.179* 10^(-18))=[(1)/(n_f^2)-(1)/(8^2)]\\\\0.0243=[(1)/(n_f^2)-(1)/(64)]\\\\0.0243+(1)/(64)=(1)/(n_f^2)\\\\0.039925=(1)/(n_f^2)\\\\n_f^2=25\\\\n_f=5](https://img.qammunity.org/2021/formulas/chemistry/college/g3npshtgg7z2s857uy1zym4nhxzlsh988q.png)
So, the final level of the electron is 5.