Answer:
See explanation
Step-by-step explanation:
For the first part of the question, we have to check the chiral carbons in 4-methyl-1,2-cyclohexanediol. In this case carbons, 1 and 2 are chiral, if we have 2 chiral carbons we will have 4 isomers. We have to remember that formula 2^n in which "n" is the number of chiral carbons, so:
2^n = 2^2 = 4 isomers
And the isomers that we can have are:
1) (1R,2S)-4-methylcyclohexane-1,2-diol
2) (1S,2S)-4-methylcyclohexane-1,2-diol
3) (1S,2S)-4-methylcyclohexane-1,2-diol
4) (1S,2R)-4-methylcyclohexane-1,2-diol
See figure 1
For the second part of the question, we have to remember that the oxidation with
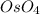 is a syn addition. In other words, the "OHs" are added in the same plane. In this case, we have the methyl group with a wedge bond, so the "OH" groups will have a dashed bond due to the steric hindrance. Due to this we only can have 1 isomer ((1S,2R,4S)-4-methylcyclohexane-1,2-diol). Finally, on this molecule, we dont have any symmetry planes (this characteristic will cancel out the optical activity), so the product of this reaction has optical activity.
is a syn addition. In other words, the "OHs" are added in the same plane. In this case, we have the methyl group with a wedge bond, so the "OH" groups will have a dashed bond due to the steric hindrance. Due to this we only can have 1 isomer ((1S,2R,4S)-4-methylcyclohexane-1,2-diol). Finally, on this molecule, we dont have any symmetry planes (this characteristic will cancel out the optical activity), so the product of this reaction has optical activity.
See figure 2
I hope it helps!