Answer:
(D) CuCl2 and Na2CO3.
Step-by-step explanation:
Hello,
In this case, we can represent the aqueous-phase reaction of D as shown below:
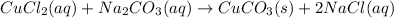
Such precipitation of copper (II) carbonate is shown off due to its tiny solubility of 8.41x10⁻⁵ mol/L or 0.0157 g/L in water. It means that only 0.0157 grams of cupper (II) carbonate are soluble in 1 liter of water. Moreover, copper salts tend to be greenish, for that reason it is a green-colored precipitate.
Unlike the aforementioned case, the other ones do not produce colored precipitates even when barium acetate and barium carbonate are not too soluble but they are colorless. On the other hand even when copper (II) acetate is colored, it is slightly soluble in water.
Best regards.