Answer:
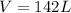
Step-by-step explanation:
Hello,
In this case, for the given reaction:
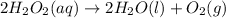
Starting with 315 g of hydrogen peroxide, we can compute the yielded moles of oxygen by using the following stoichiometric factor whereas the hydrogen peroxide to oxygen mole ratio is 2:1:
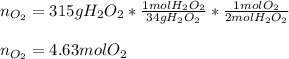
Then, by using the ideal gas equation we can compute the resulting volume if the 4.63 moles of oxygen are collected at 0.792 atm and 23 °C as shown below:

Best regards.