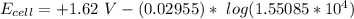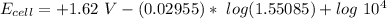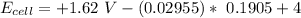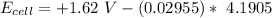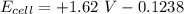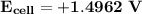HERE IS THE COMPLETE QUESTION
what is the calculated value of the cell potential at 298 k for an electrochemical cell with the following reaction, when the Cl2 pressure is 1.31 atm, the cl- concentration is at 1.28M, and the Ni2+ concentration is 1.24M
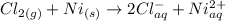
Answer:
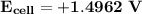
Step-by-step explanation:
From the given question;
We can see that Nickel is oxidized and Chlorine is reduced. We also known that Oxidation occurs at the anode while reduction occurs at the cathode.
SO the Oxidation and the reduction reaction can be expressed as shown below.
Oxidation :
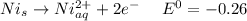
Reduction :
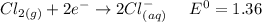
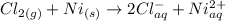
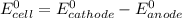
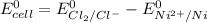
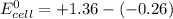
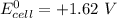
By applying Nernst Equation; we have :
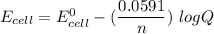
where
n= 2 moles
![Q= ([Cl^-]^2[Ni^(2+)])/(P_(Cl_2))](https://img.qammunity.org/2021/formulas/chemistry/college/6u280e392jyaws4cm4cl0muzzks1cuaphx.png)
![E_(cell) = +1.62 \ V}- ((0.0591)/(2)) \ log ([Cl^-]^2[Ni^(2+)])/(P_(Cl_2))](https://img.qammunity.org/2021/formulas/chemistry/college/8wo27rdffmrcjra8ju541ynx1eeux2n9zi.png)
![E_(cell) = +1.62 \ V}- ((0.0591)/(2)) \ log ([1.28]^2[1.24])/(1.31*10^(-4))](https://img.qammunity.org/2021/formulas/chemistry/college/488ldqst8v0yekwtgqatgb2wtvv8tytzav.png)
![E_(cell) = +1.62 \ V}- (0.02955) \ log ([1.6384][1.24])/(1.31*10^(-4))](https://img.qammunity.org/2021/formulas/chemistry/college/z8etiow297vh0xan7de3di8ekhb9bpvt90.png)
![E_(cell) = +1.62 \ V}- (0.02955) \ log ([1.6384*1.24])/(1.31)*10^(4)](https://img.qammunity.org/2021/formulas/chemistry/college/khoswpp0yehzfrzn7153n9nsq88ehjbjp4.png)
![E_(cell) = +1.62 \ V}- (0.02955) \ log ([2.031616])/(1.31)*10^(4)](https://img.qammunity.org/2021/formulas/chemistry/college/uptzcr3qk6tpuo6jz0pe8rt269qcmy0jbv.png)