Answer:
The required new pressure is 775 mm hg.
Step-by-step explanation:
We are given that gas has a volume of 185 ml and a pressure of 310 mm hg. The desired volume is 74.0 ml.
We have to find the required new pressure.
Let the required new pressure be '
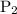 '.
'.
As we know that Boyle's law formula states that;
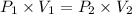
where,
 = original pressure of gas in the container = 310 mm hg
= original pressure of gas in the container = 310 mm hg
 = required new pressure
= required new pressure
 = volume of gas in the container = 185 ml
= volume of gas in the container = 185 ml
 = desired new volume of the gas = 74 ml
= desired new volume of the gas = 74 ml
So,
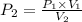
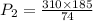
= 775 mm hg
Hence, the required new pressure is 775 mm hg.