Answer:
47.68 mL
Step-by-step explanation:
In this case, we have a dilution problem. So, we have to start with the dilution equation:
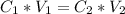
We have to remember that in a dilution procedure we go from a higher concentration to a lower one. With this in mind, We have to identify the concentration values:
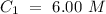
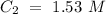
The higher concentration is C1 and the lower concentration is C2. Now, we can identify the volume values:
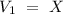
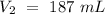
The V2 value has "mL" units, so V1 would have "mL" units also. Now, we can include all the values into the equation and solve for "V1", so:
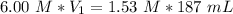
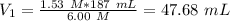
So, we have to take 47.68 mL of the 6 M and add 139.31 mL of water (187-47.68) to obtain a solution with a final concentration of 1.53 M.
I hope it helps!