Answer:
Molecules = 7.5 × 10²³ molecules
Step-by-step explanation:
Given:
Moles = 1.25 mol
Avogadro's No. =
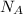 = 6.022 * 10²³
= 6.022 * 10²³
Required:
Molecules = ?
Formula:
Molecules = Moles ×
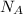
Solution:
Molecules = 1.25 × 6.022 × 10²³
Molecules = 7.5 × 10²³ molecules