Answer:
3.07 × 10⁻⁴
Step-by-step explanation:
Step 1: Calculate the concentration of H⁺
We will use the definition of pH.
![pH = -log [H^(+) ]\\\[ [H^(+) ] = antilog -pH = antilog -2.37 = 4.27 * 10^(-3) M](https://img.qammunity.org/2021/formulas/chemistry/college/67pv2qoupywgvp11az1k6ufxy02v88ltzl.png)
Step 2: Calculate the concentration of HY
5.22 × 10⁻³ mol of HY are dissolved in 0.088 L. The concentration of the acid (Ca) is:
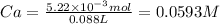
Step 3: Calculate the acid dissociation constant (Ka)
We will use the following expression.
![Ka = ([H^(+)]^(2) )/(Ca) = ((4.27 * 10^(-3) )^(2) )/(0.0593) = 3.07 * 10^(-4)](https://img.qammunity.org/2021/formulas/chemistry/college/g1iqz4n4foav2y9v32mtssd3jwfjsg3ory.png)