Answer:
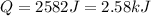
Step-by-step explanation:
Hello,
In this case, for us to compute the absorbed heat, we apply the following equation:
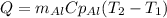
Whereas we use the mass, specific heat and temperature change for the piece of aluminium, thus, we obtain:
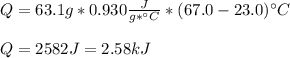
It is positive as the heat is entering, therefore the temperature raises.
Best regards.