Answer:
-
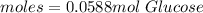
-
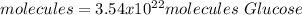
-
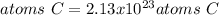
Step-by-step explanation:
Hello,
In this case, by knowing molar mass of glucose is 180.156 g/mol, we can apply the following mole-mass-particles relationships in order to compute the moles, molecules and carbon atoms in the 10.6-g sample of glucose:
- Moles:

- Molecules: we also use Avogadro's number.
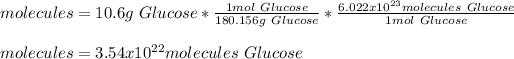
- Carbon atoms: here, particularly, one mole of glucose has six moles of carbon atoms, thus:
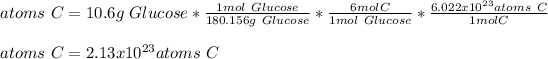
Regards.