Answer:
the initial temperature is 22.5 atm
Explanation:
The law describing pressure-temperature relationship is the Gay-Lussac's Law.
The Mathematical Form of which is:
=>
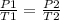
Where P1 = ? , P2 = 30 atm, T1 = 600 K and T2 = 800 K
=>
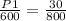
=> P1 = 0.0375 * 600
=> P1 = 22.5 atm
So, the initial temperature is 22.5 atm