Answer:
The composition of the allow, in weigh percent, is 89.971 % Titanium, 6.026 % Aluminium and 4.003 % Vanadium.
Step-by-step explanation:
The weight percentage of a element in an allow is equal to the mass of the element divided by the total mass of the allow and multiplied by 100. Then:
Titanium (Ti)
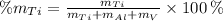
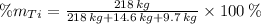
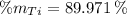
Aluminium (Al)
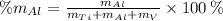
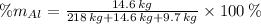
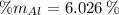
Vanadium (V)
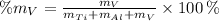
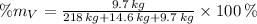
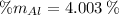
The composition of the allow, in weigh percent, is 89.971 % Titanium, 6.026 % Aluminium and 4.003 % Vanadium.