Answer : The image is attached below.
Explanation :
For
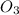 :
:
Molar mass, M = 48 g/mol
Mass, m = 24 g
Moles, n =
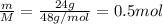
Number of particles, N =
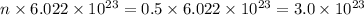
For
 :
:
Molar mass, M = 17 g/mol
Mass, m = 170 g
Moles, n =
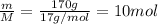
Number of particles, N =
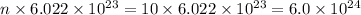
For
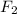 :
:
Molar mass, M = 38 g/mol
Mass, m = 38 g
Moles, n =
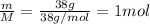
Number of particles, N =
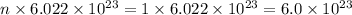
For
 :
:
Molar mass, M = 44 g/mol
Moles, n = 0.10 mol
Mass, m =
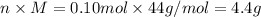
Number of particles, N =
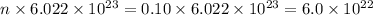
For
 :
:
Molar mass, M = 46 g/mol
Moles, n = 0.20 mol
Mass, m =
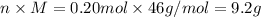
Number of particles, N =
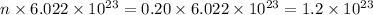
For
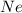 :
:
Molar mass, M = 20 g/mol
Number of particles =
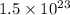
Moles, n =
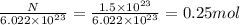
Mass, m =
For
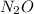 :
:
Molar mass, M = 44 g/mol
Number of particles =
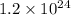
Moles, n =
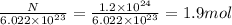
Mass, m =
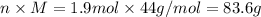
For unknown substance:
Number of particles =
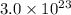
Mass, m = 8.5 g
Moles, n =
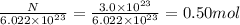
Molar mass, M =
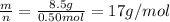
The substance is
 .
.