Answer:
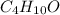
Step-by-step explanation:
Hello,
In this case, the first step is to compute the moles of carbon in the sample that are contained in CO2 only at the products as shown below:
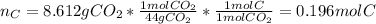
Next the moles of hydrogen contained in the H2O only:
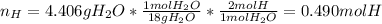
Now, we compute the mass of oxygen in the sample, by subtracting mass of both carbon and hydrogen from the 3.626 g of sample:
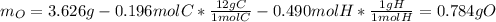
And the moles:
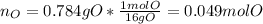
Now, the mole ratios by considering the moles of oxygen as the smallest:
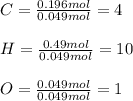
Thus, empirical formula is:
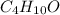
Regards.